Nonstress and Contraction Stress Testing
Authors
INTRODUCTION
Evaluation of antepartum fetal condition has become essential to obstetric care in both normal and complicated pregnancies. Many biochemical and biophysical assessment methods have been introduced during the past two decades. However, few have withstood the test of time better than fetal heart rate (FHR) testing. Of the various FHR testing schemes that have been studied, two primary methods for analyzing intrauterine fetal well-being have evolved: the nonstress test (NST) and the contraction stress test (CST). This chapter presents the physiological basis for these tests and their methodology, interpretation, and clinical application, along with illustrative examples. Pitfalls, liabilities, and unresolved issues of FHR testing are also discussed.
The concept of using FHR patterns to evaluate the fetus and fetoplacental unit is derived from earlier observations made during the intrapartum period.1, 2 The association of specific intrapartum FHR patterns with perinatal outcomes has led to the use of FHR monitoring for investigating antepartum problems. However, it should be clearly understood that observations based on the function of a single system have both diagnostic and prognostic limitations. Investigators and practitioners have tended to place increasing emphasis on the importance of individual tests,3, 4 while occasionally ignoring more global elements of pregnancy. FHR testing must be considered an ancillary aid in clinical decision making and not an ultimate answer to the complex clinical problems faced by obstetricians charged with the care of potentially compromised infants.
PHYSIOLOGICAL BASIS OF FHR TESTING
Heart rate patterns of normal fetuses reflect physiological responses to various endogenous and exogenous stimuli.5 The normal baseline record of FHR provides evidence that intrinsic control mechanisms responsible for cardiovascular autoregulation are intact. Control of FHR requires electrical conduction pathways, cellular receptors to circulating neurohormones, reflex arcs, and inherent myocardial contractility.5 The use of specific FHR patterns to evaluate fetoplacental status is based on the association of their components with particular intrauterine events or conditions. These components include baseline rate, rate variation, and episodic rate responses to fetal movements (accelerations) or uterine contractions (decelerations). The characteristics of these FHR components are determined by both cellular and systemic mechanisms.
FHR patterns and cellular events
The functional units of the fetal heart are myocardial fibers that act as a syncytium; they are endowed with inherent rhythmicity, apparent from the first trimester onward.6 Cellular events within the myocardium are influenced by oxygen supply, energy substrates, membrane receptors to circulating hormones, and preservation of cell integrity. Cell growth or hypertrophy is a dominant feature of cardiac development in the final trimester of pregnancy, during which most FHR testing is performed. This process is energy consuming and requires adequate transport of oxygen, glucose, and amino acids.
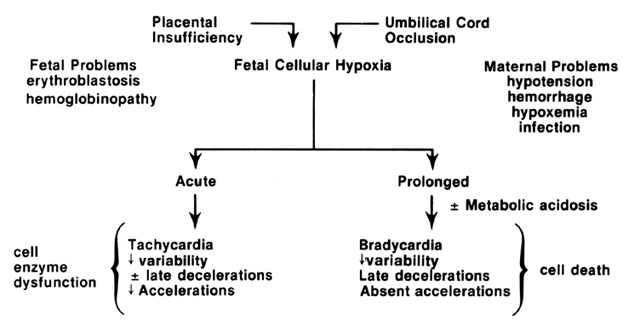
Under normal conditions, the placenta serves as a respiratory and nutritive organ. Nutritive functions, maintained throughout pregnancy, lead to a positive balance of glucose. Eventual glycogen deposition in cardiac and hepatic tissue provides a reservoir for the stresses of parturition and early neonatal life. Under adverse circumstances (e.g., decreased utero-placental perfusion or maternal malnutrition), these stores may be prematurely exhausted; consequently, fetal growth and energy-dependent biophysical activities are curtailed. Placental respiratory failure may alter cellular metabolism. Increased tissue extraction of oxygen from high-affinity fetal hemoglobin may offer short-term protection from this problem. However, the inability of the placenta to exchange oxygen and carbon dioxide results in fetal respiratory acidosis. Excess hydrogen ions accumulate in fetal circulation; progressive cellular hypoxia and diminished aerobic metabolism result in development of a secondary metabolic acidosis. Critical intracellular enzymatic reactions begin to fail, and glucose is broken down to lactate and pyruvate, augmenting metabolic acidosis. Failure to interrupt this sequence of events may lead to cellular death, reduced myocardial contractility, and inability to maintain systemic homeostasis.
The relationship of cellular events to the pathophysiology of FHR tracings is summarized in Figure 1. Although there is controversy regarding the earliest FHR manifestations of cellular hypoxia and tissue acidosis, their expression will depend on both the chronicity and severity of the actual insults and may not be uniformly appreciated by all compromised fetuses. The ultimate or preterminal patterns associated with cellular hypoxia and systemic asphyxia consist in relatively fixed FHR baselines, reduced or absent FHR variation, absence of FHR accelerations, and the appearance of spontaneous late FHR decelerations.7, 8
FHR and systemic influences
Unstressed or resting FHR tracings are indices of the following: (1) parasympathetic (vagal) tone; (2) sensitivity to sympathetic (adrenergic) discharge; (3) organization of fetal activity or behavioral states; (4) circadian rhythms; (5) the linkage between body movements and accelerations; (6) increasing stroke volume with reduction in resting or intrinsic rate; and (7) integration of reflex responses to momentary fluctuations in arterial blood pressure and gas partial pressures.
These complex interrelationships (Fig. 2) are also affected by maturational effects, maternal state factors, and pathologic conditions. FHR accelerative responses are regulated through accelerator nerve fibers arising in the upper thoracic roots and are fine-tuned in the hypothalamic and medullary regions of the brain, which are sensitive to momentary changes in oxygen tension, acid-base balance, circulating catecholamines, and endorphins. These pathways are evident as early as the midtrimester.6 FHR decelerative responses are partly generated through parasympathetic pathways, resulting from direct vagal stimulation and baroreceptor and chemoreceptor arcs (aortic arch, carotid sinus).9
Fetal movements become increasingly frequent in the midtrimester and act as triggers for transient baseline alterations with stronger linkages as term is approached.10, 11 Natale and co-workers10 have shown that from 24 to 32 weeks, despite a gradual decrease in the incidence of fetal movements, the association of accelerations with movements increases and the amplitude and duration of accelerations become greater. Others11, 12 have reported that the frequency of movement-associated decelerations decreases with gestational age, especially after 29–32 weeks.
Behavioral organization becomes more important in the late third trimester, since clustering of movements and accelerations become more apparent during this general time frame.13, 14 Animal studies suggest that the increasing tendency to generate accelerations with body movements may also result from increased myocardial sensitivity to endogenous catecholamines, presumably a reflection of increasing numbers of myocardial receptor sites.15
The element of time plays a greater role in the occurrence of FHR patterns as fetal cardiovascular control systems mature. Nonrandom, periodic cycles of FHR are generated, lasting from 60 to 500 minutes,16 with a mean duration of approximately 90 minutes at term. As state organization becomes better defined, epochs of low variability and decreased movement incidence recur, lasting as long as 90–120 minutes, with a mean of approximately 20 minutes.17 These sequences alternate, through transitions, with periods of increased fetal breathing and rapid eye movements associated with occasional body movements. These states of active sleep occupy 40–60% of the average 24-hour day18 and are also reflected by increased baseline FHR variability. Brief episodes of “wakeful” activity, approximately 10–15% of the total day, account for the majority of epochs during which reactive FHR accelerations are observed. Diurnal fluctuations in body movements have been reported, with peaks occurring between 0100 and 0700 hours.19 Throughout the day, mean frequency of FHR accelerations exceeding 15 beats per minute amplitude is 15–20 per hour.17
Maternal factors may influence the patterns present in resting FHR tracings. Maternal fed state, responsible for maternal glycemia, has had an inconsistent effect on reported FHR responses.20, 21 These reports failed to demonstrate an increase in either fetal movement or acceleration frequency following maternal glucose loading. Maternal activity levels22 are also associated with a variety of FHR patterns. Normal ambulation23 appears to have little appreciable influence on either subsequent baseline rate or acceleration incidence; graded vigorous exercise may cause transient but unpredictable tachycardias and bradycardias.22 Numerous drugs administered to pregnant women near term have been studied.24 β-blocking agents such as propranolol tend to lower mean baseline FHR and reduce acceleration frequency.25, 26 Long-acting central nervous system depressants may theoretically increase the time required for eliciting reactive accelerations,27 whereas nicotine may transiently elevate baseline rate, reduce uteroplacental perfusion, and delay the onset of a normal reactive pattern.28
Systemic influences on resting FHR tracings due to ongoing maternal or fetoplacental pathology have a common pathway in which oxygenation and energy substrates are reduced. In the absence of acidosis, acute disturbances of placental respiratory or nutritive function may result in sudden and profound decrease in fetal movement incidence. The corollary to this situation would be marked decreases in acceleration frequency. More commonly, diminished placental functions are more subtle, tend to be chronic, and lead to gradual declines in fetal movement incidence and acceleration frequencies as compensatory visceral shunting of the fetal circulation occurs.29, 30 Consequently, days or weeks may elapse before the impact of chronic placental failure can be appreciated from alterations in resting FHR patterns.
Uterine activity and FHR patterns
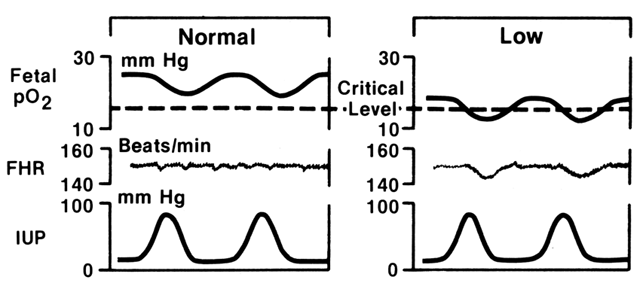
As term pregnancy approaches, the frequency of spontaneous uterine activity increases and individual contractions tend to become longer and more intense. Early data, using direct measurements of intrauterine pressure, suggest that a contraction intensity of more than 35 mmHg is needed before the effects of transient hypoxia are consistently appreciated by borderline or compromised fetuses.1 The concept of gauging the impact of such uterine activity on basal fetal oxygenation and subsequent alteration of FHR is illustrated in Figure 3. During any given contraction of moderate to strong intensity, intervillous space blood flow is greatly reduced or abolished, restricting oxygenation of the fetus. The hypoxemic fetus tolerates such stress poorly, and myocardial homeostasis becomes insufficient to maintain effective cardiac output during this period. The resulting late FHR decelerations have both a reflex component (i.e., vagotonic in origin) and a component directly related to myocardial depression. In general, the depth and duration of this response are reflections of the intensity and duration of the uterine contraction itself, whereas the “lag” between the peak of the contraction and nadir of the deceleration is a reflection of the pre-existing level of myocardial depression. It should be carefully noted that other mechanisms may be responsible for late FHR decelerations: (1) intrinsic maternal hypoxemia (respiratory disease, anemia); (2) maternal hypotension (aortocaval compression, drugs); or (3) compromise of umbilical venous blood flow (partial cord occlusion).
Uterine activity may also be associated with fetal movements and FHR accelerations.31 These FHR alterations occur commonly during labor and are typically associated with a healthy, well-oxygenated fetus.32 Unless the fetus is being directly visualized during periods of uterine activity, it may not be possible to distinguish accelerations associated with fetal movements from those associated with mild cord compression. Therefore, this clinical correlation should be interpreted cautiously during antepartum monitoring.
NONSTRESS TESTING
Historical background
Nearly 40 years ago, Hammacher observed that “the fetus can be regarded as safe, especially if reflex movements are accompanied by an obvious increase in the amplitude of oscillations and in the baseline fetal heart rate.”33 This study formed the basis for the NST and underscored the important association of FHR accelerations with fetal health. The NST was introduced to the USA nearly 10 years later through the work of Lee and associates34 and Rochard and co-workers35 who developed clinical testing schemes based on resting FHR tracings. Subsequently, more than 100 studies of the NST have appeared in English language literature and numerous approaches for using this test have been evaluated. Most NST schemes use minimum thresholds of FHR acceleration frequency to distinguish healthy from compromised fetuses. As is discussed later, the value of “reactivity” or accelerations associated with fetal movement may vary considerably with the composition of the population tested, gestational age, the frequency of test repetition, and the use of other baseline FHR features in test evaluation, including the use of extended testing sessions36, 37 and extension to earlier-gestational age categories.38, 39, 40
Technique/methodology
INSTRUMENTATION
Most obstetric laboratories now use FHR transducers operating in either continuous or pulsed Doppler modes rather than phonocardiographic or abdominal electrocardiographic signal sources. Advances in Doppler signal processing, using onboard autocorrelation techniques,41, 42 have produced legible FHR tracings that appear similar to those obtained from direct fetal scalp electrode sources. However, external FHR signals generated in this manner do not represent true electrocardiographic R-R intervals. Consequently, valid “beat-to-beat” or short-term FHR variability cannot be directly determined using this method. Nevertheless, the tendency of these systems to exaggerate baseline variability through artifactual “jitter” has been greatly reduced.41 A tracing with minimal or absent baseline fluctuations is cause for some concern, regardless of the instrumentation used to capture the FHR signal.
External tokodynamometer devices are used to register uterine activity. These transducers are sensitive to changes in surface abdominal wall tension, and their reliability and relationship to actual intrauterine pressure readings have been well studied.43 Technical problems may arise during the recording of uterine activity under conditions of uterine overdistention or maternal obesity. The validity of using patient-operated markers for fetal activity is dependent on the quality of patient involvement and education, and corroboration by experienced observers. Under best circumstances, a better than 90% correlation between perceived and actual fetal movements can be achieved.44
Points to be emphasized during performance of the NST include uniformity of testing conditions, length of observation, consideration of maternal status, and selection of high-fidelity recording equipment. Fetuses are often tested on more than one occasion, emphasizing the need for careful control of such factors as time of day, maternal activity levels, medication and dietary status, and observation techniques if serial comparisons of tests are to be considered in management protocols. Although no minimum length of testing has been universally accepted, extremely short intervals (10 minutes or less) may result in interpretative and classification errors for normal fetuses.45 Conversely, extending tests for as long as 90–120 minutes may be necessary in situations in which reactive accelerations are absent secondary to prolonged physiologic sleep states or immaturity.36, 37 Close attention needs to be paid to maternal status during testing. Maternal positioning should prevent aortocaval compression, vital signs should be recorded every 10–15 minutes, and any unusual events such as audible FHR irregularities should be noted. Finally, the quality of recorded signals is a limiting factor for interpretation. It is of paramount importance if the NST is to be a useful screening or diagnostic test. Fortunately, most current operating systems are capable of achieving excellent-quality tracings, have wide-range probes, and are relatively tolerant of shifts in fetal position. The testing protocol used at the Medical College of Georgia is outlined in Table 1.
Table 1. Testing protocol for NST (Medical College of Georgia)
- Equipment: electronic fetal-maternal monitor
FHR: Doppler signal source
UC: external tokodynamometer + manual palpation
FM: remote event marker + observer confirmation - Time of day: 0800–1600 h
- Maternal fed state: 2 h postprandial
- Maternal activity: 2 h sedentary
- Maternal position: semi-Fowler's, lateral hip displacement
- No smoking/sedative drug within 2 h
- Baseline observation period: 30 min
FETAL STIMULATION MANEUVERS: VIBROACOUSTIC STIMULATION
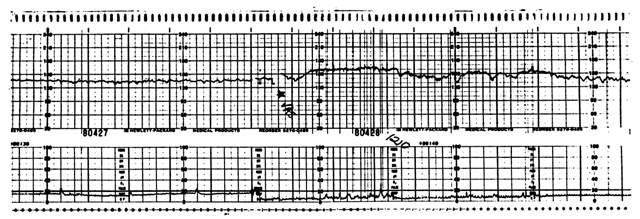
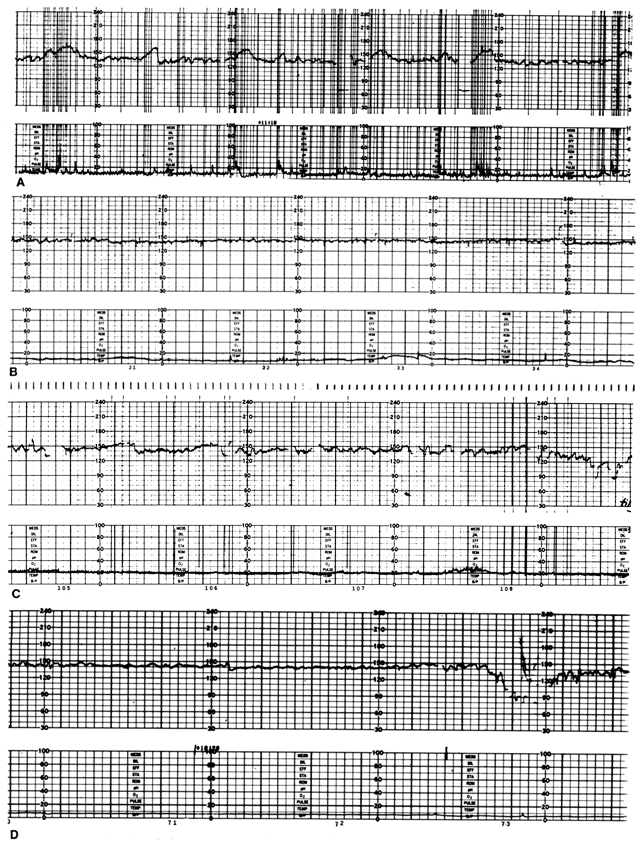
In some centers, protocols have included procedures to induce “reactivity” in the baseline FHR pattern. Earlier approaches had advocated manual palpation or shaking of the fetal head or body. However, these maneuvers have not consistently elicited more frequent accelerations or led to shorter testing times.46 Vibroacoustic stimulation (VAS) of the fetus has been used as both a primary and adjunctive method of FHR testing. Initial studies used pure tone generators.47, 48 Subsequently, there has been extensive evaluation of vibroacoustic stimulation using an electronic artificial larynx. This device produces a broadband acoustic signal and a complex vibratory component. Smith and associates performed a retrospective survey of this approach during antepartum testing and showed that the use of VAS reduced the occurrence of nonreactive tests from 12% to 6%.48 Their later prospective study49 confirmed these results and suggested that a reactive FHR tracing, whether occurring spontaneously or after VAS, conferred reliable assessment of fetal well-being.49 These authors' techniques have been used in most centers that have adopted VAS. The stimulator is applied to the maternal abdomen in the region of the fetal head, then a 3-second pulse is delivered. A sample VAS-evoked reactive test is shown in Figure 4.
|
The most significant factor that influences fetal response to VAS appears to be gestational age. Gagnon and co-workers50 showed that, as the fetus matures, there is increased consistency of response to VAS, in terms of increased body and breathing movements, suggesting that this stimulus may produce a change in organized fetal behavioral state. Subsequently, Devoe and colleagues51 confirmed with real-time ultrasound and simultaneous FHR recordings that behavioral states may be altered by VAS, primarily when fetuses are in quiet sleep (state 1F). In an additional study,52 this group quantitated the typical responses of healthy term fetuses to VAS. Their data showed that nearly all such fetuses will respond to a standard stimulus with at least a 10 bpm rise in baseline, occurring in about 7–10 seconds and lasting from 5–10 minutes. The failure to generate a reactive VAS test was rare in healthy subjects, occurring in about 2% of tests.
While VAS promises to effect a test with similar predictive value in a potentially shorter time frame, there has been concern about its safety and long-term sequelae. Unfortunately, there are few direct data to address these issues. In utero sound pressure levels have been measured with specially adapted hydrophones, yielding stimulus peaks ranging from 98 to 111 db.53 While sound at such intensity for prolonged periods could injure hearing, such brief exposures have not proved to be harmful to the 500 children whose hearing acuity and general neurologic development have been studied to date.54
FETAL ACTOCARDIOGRAPHY: FETAL MOVEMENT AND HEART RATE TESTING
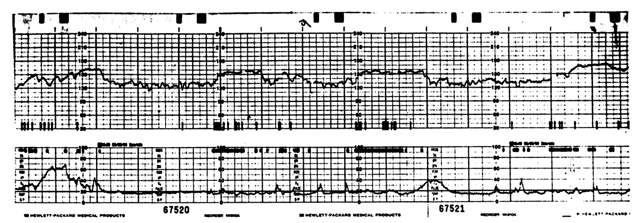
Adaptations of Doppler signal processing enabled the development of a method of antepartum monitoring which combines the simultaneous detection of FHR and fetal body movements. This approach takes advantage of special band-pass filters which, when applied to the raw Doppler signal, allow isolation of the low frequency shifts associated with fetal movement from the higher frequency alterations associated with fetal cardiac motion. A number of groups have developed monitoring systems that display these data simultaneously55, 56 (Fig. 5). Besinger and Johnson57 and Melendez and associates58 have both shown that these systems produce a Doppler detection of fetal activity, which is strongly correlated with ultrasound-visualized fetal movements in about 85–93% of instances.
Stanco and co-workers59 showed that the use of actocardiography in antepartum testing decreased significantly (5.7–3.3%) the incidence of tests interpreted as “nonreactive”. A large survey of actocardiography compared the predictive values of Doppler movement detection with standard reactivity parameters of the NST.60 It concluded that quantitation of time spent in active movement (fetal movement percentage) was as good at detecting compromised and healthy fetuses as the more traditional measure of FHR accelerations. Further, Doppler detection of fetal movements acquired about 100% more movements than those resulting from concurrent maternal perception.
Application of actocardiography to actual fetal assessment should still be considered investigational. The additional data obtained from detecting fetal movements would appear to aid in the distinction of true and false nonreactive tests, and help to distinguish changes in fetal behavioral state.60 However, more studies are required before the method can be considered as reliably established as the standard NST.
AUTOMATED ANALYSIS OF THE NST: COMPUTERIZED SYSTEMS
There have been several efforts to assist the analysis of the NST by the application of computerized systems. We have acquired substantial clinical experience with our own system (NST-ANALYST)61 and that developed by Dawes and colleagues (Oxford Sonicaid System 8000).62 Both systems function in a similar manner: the processed FHR signal is ported from a standard electronic monitor to a programmed desktop computer. Once acquired by the computer, FHR baseline, its variation, accelerations, decelerations, and signal loss are computed and rated against established population standards.
While considerable time has been expended on the development and description of these systems, sufficient trials of clinical efficacy are yet to be undertaken. Preliminary studies have compared the output of the System 8000 to conventional NST assessment.63, 64 These independent investigations concluded that such systems, designed to be consistent and objective, could serve in primary screening when experts are not immediately available. Further, there is a suggestion that such approaches are potentially time- and effort-saving and might reduce the necessity for additional evaluation methods.64, 65
INDICATIONS AND CONTRAINDICATIONS
All pregnant women at risk for intrauterine compromise may be considered as candidates for nonstress testing. Common examples of risk include prolonged pregnancy; maternal hypertensive disorders; intrauterine growth retardation; diabetes mellitus; Rh sensitization; maternal hemoglobinopathies; renal disease; cardiac disease; fetal anomalies; poor prior obstetric history; and reported decrease in perceived fetal movement. No absolute or major relative contraindications for the NST have been identified.
NST INTERPRETATION
Many interpretative criteria have been developed; representative examples of these are found in Table 2. Most testing schemes use a fixed threshold for a minimum frequency of FHR accelerations in a given length of observation. Considerable disagreement persists as to the minimum threshold for acceleration recognition (i.e., amplitude of 10 beats/min vs 15 beats/min) and the inclusion of additional FHR baseline information (e.g., long-term variability, rate, presence of occasional spontaneous decelerations). The most frequently used classification system for FHR reactivity considers the presence of at least two accelerations exceeding an amplitude of 15 beats/min and a duration of 15 seconds during a 20-minute epoch.66, 67 Critical reviews of testing standards and diagnostic values reveal that a wide range of test sensitivity, specificity, and predictive values is associated with any given cutoff point4 and that the applicability of arbitrary reactivity criteria may depend on the prevalence of poor fetal outcomes in test populations. Therefore, interpretation of the NST may be generalized to include the occurrence of at least one FHR acceleration with movement during the baseline period,68 a normal baseline FHR,69 the absence of periodic or spontaneous FHR decelerations,70 and, in some schemes, the presence of “normal” baseline variability.71, 72, 73 An NST may be nonreactive if accelerations are absent or subnormal in their dimensions (amplitude, duration) and fetal movements are absent or do not elicit FHR accelerations. An NST may be “abnormal” if any of the above obtain and the test is accompanied by sustained tachycardia (rate >160 beats/min) or bradycardia (rate <120 beats/min), decreased or absent baseline oscillations, periodic late or variable decelerations, spontaneous decelerations or bradycardias, or fetal arrhythmia. Sequential studies of NSTs in the same fetus74 suggest that the evolution of fetal compromise may begin with a gradual decrease in the frequency of accelerations, a subsequent decrease in the incidence of fetal movement, a decoupling of accelerations with fetal movements, and the disappearance of both accelerations and movements. Examples of typical NST tracings are presented in Figure 6.
Table 2. NST interpretative criteria
Study (Year) | Baseline Period (Minutes) | Acceleration No. (Amplitude) | Other Baseline Alterations |
Lee (1976) | 15 | 4 (10, 15) | No |
Rochard (1976) |
| (10) | Variability |
Schifrin (1979) | 10 | 2 (15) | No |
Evertson (1979) | 20 | 5 (15) | No |
Devoe (1980) | 30 | 3 (15) | No |
Flynn (1979) | 20 | 4 (15) | Variability: decelerations |
Brown (1981) | Up to 120 | 5 (15) | No |
Mendenhall (1980) | 30 | 1 (10) | No |
Krebs (1978) | 30 | 5 parameter score |
|
Visser (1977) | 20–30 | 4 patterns unscored |
|
Aladjem (1981) | 30 | % fetal movement + accelerations Total fetal movement >51 |
|
Devoe (1986) | 30 | Total acceleration time Total test time >5 |
|
Clinical considerations
The NST has become useful in antenatal surveillance because of wide applicability, ease of performance, and relatively low cost.75 Its ability to produce rapid results enables testing centers to screen high volumes of at-risk pregnancies efficiently. The NST is an effective approach for evaluating a wide range of potential antenatal problems, including intrauterine growth retardation (IUGR),76, 77 prolonged gestation,78, 79 preterm pregnancy,38, 80 multiple gestation,81, 82, 83 Rh sensitization,35 and anomalies.84, 85
As a primary assessment tool, the NST has been suboptimal in the detection of IUGR, as many of these fetuses will continue to exhibit FHR reactivity in the face of abnormal fetal growth.77 Risk assessment in prolonged pregnancy has been complicated by the relatively low frequency of truly postmature infants and the fact that highest perinatal risk occurs during the intrapartum period;86 consequently, a falsely reassuring test may precede the occurrence of intrapartum fetal distress or meconium aspiration. Clinical studies of fetuses between 24 and 32 weeks' gestational age have found distinct maturational trends in FHR patterns, suggesting that interpretative criteria different from those used near term should be considered. Reactivity in preterm fetuses may be characterized by a higher incidence of low amplitude (10–15 beats/min) accelerations,10 weaker coupling between fetal movements and accelerations,11 and more frequent mild decelerations.10 Devoe and Azor demonstrated that simultaneous nonstress testing of twin pregnancy was feasible with a high rate of legible recordings.82 Rh sensitization presents a relatively unique antenatal problem for FHR testing in that fetal problems include reduced oxygen-carrying capacity, umbilical cord compression secondary to hepatomegaly, and intravascular volume disturbances. A feature of FHR testing peculiar to this condition is the so-called sinusoidal pattern, which is characterized by repetitive low-amplitude, uniform oscillations, usually without reactive accelerations. Fetuses exhibiting this pattern appear to be at extremely high risk for morbidity and mortality.87 Finally, reports of fetuses with a variety of congenital malformations have indicated that many will exhibit abnormal FHR patterns during antepartum testing.84, 85 No specific pattern has been linked with any given anomaly, although nonreactivity in excess of 2 hours, with or without spontaneous decelerations, should prompt an ultrasonographic survey for malformations.
MANAGEMENT
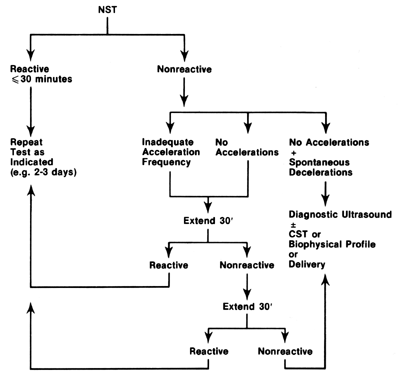
Regardless of specific interpretative criteria, several considerations are important for proper utilization of the NST: (1) gestational age at test initiation, (2) frequency of test repetition, and (3) mode of test follow-up. A generalized management scheme is shown in Figure 7. As suggested previously, gestational age at initial testing will depend on when the clinical problem is suspected. Practical considerations dictate that the NST not be used prior to the age at which neonatal survival is possible. This cutoff will vary among centers but will generally not occur prior to 25 or 26 weeks. It is also important to remember that interpretative standards different from those used at term should be applied to these earlier gestational ages.
The frequency of testing may vary according to specific high-risk indications. Current data suggest that intervals between tests should be less than 7 days88 and that the predictive power of the NST declines as the interval from last test to delivery becomes longer.41 For most clinical conditions, two to three sessions each week are recommended, with individualized schedules for patients with more severe or unstable problems (e.g., brittle diabetes mellitus).
FOLLOW-UP OF TEST RESULTS
Management of the nonreactive NST has varied considerably among testing centers. Generally, NST nonreactivity becomes significant if it persists for more than 80–120 minutes,36, 37 provided that no confounding factors, such as maternal drug administration, profound hypoglycemia, or fetal arrhythmias, are also present. Recommended follow-up of such tests include the CST, biophysical profile, or diagnostic ultrasonography to rule out congenital malformations. When spontaneous FHR decelerations occur during nonstress testing, either oligohydramnios or fetal malformations may be present, and subsequent ultrasonographic study of the fetus is suggested.
CONTRACTION STRESS TESTING
Historical background
The earlier observations of Pose and co-workers1 encouraged initial studies of antepartum FHR responses to exogenous oxytocin infusion. Ray and others,89 in a preliminary report, found that the absence of late FHR decelerations during induced uterine contractions predicted good fetal condition, whereas the appearance of repetitive late decelerations was strongly associated with stillbirths or neonatal compromise. Freeman,90 in a larger series of patients, confirmed the reliability of the CST for detecting fetuses at high risk for uteroplacental insufficiency. The concept of adding baseline reactivity to the classification of CST patterns was introduced by Trierweiler and others91 and subsequently supported by numerous studies.92, 93, 94 Huddleston and associates introduced the use of nipple stimulation, in place of intravenous oxytocin, as a means of eliciting uterine contractions in antepartum FHR testing.95 Currently, most CST schemes employ a diagnostic window of uterine activity and associated FHR baseline changes, ranging from 10 to 30 minutes, as the basis for test interpretation. Although the CST was originally introduced as a primary method of fetal surveillance, many obstetric units are now using this test selectively or as a back-up to the NST.96, 97, 98, 99, 100
Technique/methodology
INSTRUMENTATION
The CST uses the same electronic monitoring systems for FHR and uterine activity as described in the preceding section on nonstress testing. Although it may be conducted in a similar testing center, the use of intravenous oxytocin may necessitate its being performed in or near a labor/delivery suite. There are two methods of inducing uterine contractions: intravenous infusion of oxytocin and maternal nipple stimulation. Before describing these techniques, several key points should be noted. First, a baseline FHR tracing should be obtained prior to initiating the CST so that the presence or absence of spontaneous uterine contractions can be determined; if abnormal pre-existing FHR patterns are observed, further uterine stimulation may then be avoided. Next, careful monitoring of uterine activity is essential so that an adequate intensity and frequency of contractions can be achieved. This requires frequent palpation of the uterine fundus and accurate placement of the tokodynamometer. If exogenous oxytocin is to be used, a rate-controlled infusion pump should be employed so that inadvertent drug overdose may be prevented. Finally, if the nipple massage technique is chosen, an adequate rest period between stimulation sessions is desirable to decrease the possibility of uterine hyperstimulation.
INTRAVENOUS OXYTOCIN INFUSION
After a baseline record of FHR and uterine activity is obtained, it should be reviewed to determine that uterine contractions are occurring less frequently than three in 10 minutes and that no pathologic FHR alterations are present. An intravenous line is then started with normal saline and oxytocin infusion begun through an auxiliary or “piggyback” line at an initial rate of 0.5 mU per minute. The rate of infusion is controlled through a user-adjusted pump controller and should be doubled no more often than every 20–30 minutes until either a satisfactory uterine activity pattern is achieved (i.e., three moderate to strong contractions in 10 minutes) or an infusion rate of 20 mU per minute has been reached. Infusion rates exceeding 20 mU per minute are rarely needed; in such instances, further adjustment of oxytocin infusion rates should be individualized. The test is concluded when the desired uterine activity has been sustained for at least 10 minutes. At this point, the oxytocin infusion may be discontinued and the FHR and uterine activity monitored continuously until contractions are more than 10 minutes apart. Should hyperstimulation occur (i.e., tetanic contractions or tachysystole), oxytocin should be immediately discontinued and the patient placed on her side and given oxytocin by mask until uterine activity subsides. Regardless of FHR responses during hyperstimulation, a rest period of at least 1–2 hours is recommended if further contraction stress testing is to be performed.
NIPPLE STIMULATION
After a similar baseline tracing is performed, the patient is instructed to perform gentle massage on the exposed nipple of one breast with the palmar surface of her fingers or a moist cloth; this is continued for 2 minutes, followed by a 5-minute rest period. If uterine contractions ensue, no further nipple massage may be needed. This stimulation-rest cycle can be repeated until either an adequate contraction pattern is established or it is judged unsuccessful. At this time, an intravenous oxytocin infusion could be initiated.
As is true of intravenous oxytocin infusion, this technique has been successfully used in a variety of clinical settings. The advantages of nipple stimulation CST include avoidance of intravenous lines and exogenous drugs, ease of administration, and decrease in the length of time required to achieve a satisfactory tracing. The disadvantages include unpredictability of uterine response, higher failure rates, higher rates of hyperstimulation, and unknown mechanism of action.101
INDICATIONS AND CONTRAINDICATIONS
Test indications are similar to those of the NST. However, absolute and relative contraindications to the CST have been established. Absolute contraindications include preterm rupture of membranes; third-trimester bleeding, especially if due to placenta previa; prior classic cesarean section; and known hypersensitivity to oxytocin. Relative contraindications include previous preterm labor; polyhydramnios or marked uterine overdistention; and conditions that interfere with adequate uterine monitoring (e.g., marked obesity).
CST INTERPRETATION
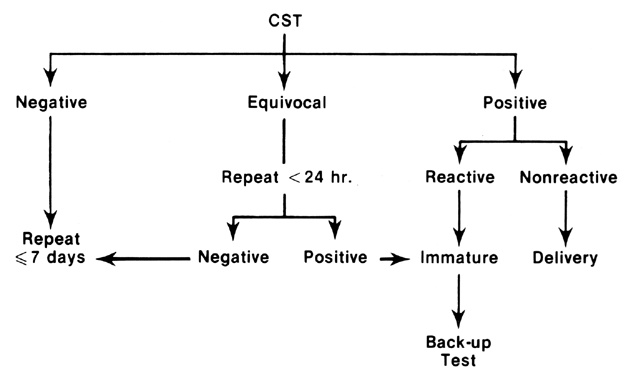
The commonly accepted categories of CST interpretation are presented in Table 3. Most interpretative criteria require that a test be conducted until a satisfactory pattern of uterine activity is established for a minimum of 10 minutes, although many centers require that it be sustained for 30 minutes.
TABLE 3. CST interpretative criteria
Result | Description |
Negative | No late deceleration(s) present on tracing with uterine activity that is adequate |
Positive | Late decelerations present with most (more than half) of the uterine contractions (unless hypertension present), even if uterine activity is less than adequate |
Suspicious | Adequate uterine activity present with some late deceleration(s), but does not meet criteria for a positive test |
Hyperstimulation | Late deceleration(s) present with or following excessive uterine activity |
Unsatisfactory | Quality of tracing inadequate for accurate interpretation or adequate uterine activity cannot be achieved |
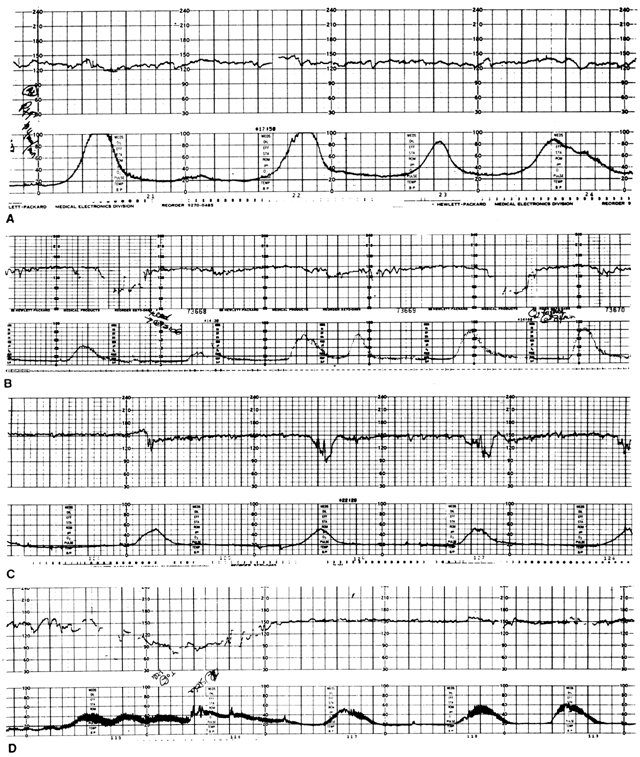
Negative CST
A negative CST (Fig. 8A) implies that no late decelerations have been present during testing. It has been considered a reliable marker of fetal well-being, with a corrected antenatal mortality rate of only 0.3/1000 and perinatal death rate of 2.3/1000.102 It has not been established that the nature of FHR baseline reactivity in an otherwise negative CST alters the prognostic capability of this test.
Positive CST
A positive CST (Fig. 8B) conveys the strong possibility that placental respiratory insufficiency is present, although it does not indicate the probable duration or progress of this condition. By widely accepted standards, a positive test implies that the majority of uterine contractions in the diagnostic window are associated with late FHR decelerations. Positive CSTs account for fewer than 10% of total tests performed in high-risk populations,102 but they are associated with corrected perinatal mortality rates of 75–100/1000, nearly equally divided between antepartum and neonatal periods. In addition, the positive CST conveys much higher risk of fetal distress, low 5-minute Apgar scores, and IUGR than does a negative test.102 FHR baseline reactivity characteristics of the positive CST appear to improve the discrimination of high-risk fetuses into two groups. Those with a reactive baseline are at much lower risk for intrauterine or neonatal compromise, while those with nonreactive CSTs constitute the highest risk group within the populations studied to date.91, 92, 93, 94 It is probable that a number of “false” positive tests occur in the former subgroup, and, as suggested by Devoe,103 only 10% developed evidence of intrapartum fetal distress when vaginal delivery was attempted.
Equivocal (suspicious) CST
An equivocal CST is a special category of test outcome in which occasional but not persistent late FHR decelerations are present. Staisch and others104 and Baskett and Sandy105 were unable to show significant differences in fetal outcome between suspicious CSTs and negative CSTs. These findings were also supported by the data of Freeman and co-workers in a collaborative study.102 This category can be eliminated in most cases by extending the period of testing until a clearly positive or negative diagnostic window is obtained.106 Such test clarification should take place either at the same testing session or within 24 hours of the original results. It is probable that most of these fetuses are well adapted, and, as shown by Trimbos and Keirse, approximately 7% of normal fetuses will exhibit one or more FHR abnormalities during the antepartum period.107
Isolated or recurrent variable FHR decelerations may occur during the CST (Fig. 8C) and are associated with a high incidence of umbilical cord complications in the antepartum or intrapartum period.108, 109, 110, 111 These pregnancies are more likely to be complicated by oligohydramnios secondary to IUGR or postmaturity. This finding during the CST should prompt ultrasound examination for amniotic fluid volume, or umbilical cord localization. In itself, this is not a cause for urgent intervention, but Druzin and associates112 have reported that the progression of this pattern to overt prolonged bradycardia carries a much more serious prognosis and may require expeditious delivery.
Hyperstimulation and unsatisfactory tests
Unsatisfactory outcomes (Fig. 8D) may be considered as testing failures in that they are neither reassuring nor clinically useful. Fortunately, in most series, they constitute less than 3% of all test results. Hyperstimulation may occur as either tetanic contractions or tachysystole and requires either a subsequent attempt at testing, after a recovery period has been completed, or selection of another fetal assessment method.113
Clinical considerations
The same types of management concerns exist for the CST as were discussed in the NST section. An overall scheme for the variety of test outcomes described earlier is presented in Figure 9. Gestational age at the initiation of contraction stress testing depends not only on clinical needs but also must be carefully considered in the early third trimester when uterine sensitivity to oxytocin is reduced. Both Gabbe and associates114 and Devoe38 have shown that a positive test, occurring between 25 and 34 weeks' gestational age, carries a similar prognosis to that obtained near term.
Following the recommendation of Freeman,90 a testing interval of 1 week following a negative CST has been adopted in many centers. Subsequent reports of fetal death during this intervening period115, 116, 117, 118 must be considered as rare failures of detection, since the majority were due to unanticipated obstetric accidents. In certain selected high-risk situations, such as unstable diabetes mellitus or hypertension, severe IUGR, or prior unexplained fetal death during a test-free interval, testing might be performed more frequently.
Clinical follow-up of the positive CST can be modified by two additional factors: fetal maturity status and the presence or absence of reactive baseline patterns. If fetal pulmonary maturity has been established, delivery should be considered whether or not reactive accelerations are present. Compared with fetuses with negative CSTs, these fetuses are at increased risk for poor outcomes, and, at the very least, delivery will avoid the possibility of continued intrauterine jeopardy. Delivery route in the presence of a positive test should be individualized. If the cervix is favorable, early amniotomy, direct scalp electrode placement, baseline evaluation of scalp blood pH, and maternal oxygenation should be instituted. These procedures permit a more reliable tracking of the FHR, assessment of acid-base balance, potential shortening of the latent phase of labor, and evaluation for the presence of meconium staining of the amniotic fluid. If the cervix is unfavorable, the presenting part unengaged, and rapid induction to delivery time improbable, the CST may be “extended” as a prelude to induction of labor. However, should late FHR decelerations persist, with the absence of baseline reactivity, cesarean delivery is a judicious management option.
If the fetus is immature or a reactive baseline pattern is present, corroboration with another assessment method is recommended prior to termination of pregnancy. Prolongation of pregnancy sufficient to permit adequate maternal glucocorticoid therapy119 should be considered only if extremely close and continuous fetal surveillance is possible for at least 48 hours. A nonreactive positive test in either a mature or an immature fetus should be considered as an indication for prompt delivery, route dependent on the condition of the cervix and feasibility of direct electronic monitoring and scalp blood sampling. Using a selective approach, Bissonnette and colleagues,120 Odendaal,121 and Devoe103 have shown that a number of fetuses with positive CSTs can be successfully delivered vaginally.
SELECTION OF A PRIMARY FHR TEST
Throughout the past decade, there has been continuous controversy over the most effective approach for primary FHR testing. Efforts to resolve this problem have polarized the community of physicians engaged in FHR testing, since both major schemes have acquired strong advocacy. No truly definitive study containing sufficient numbers of randomized patients, adequately matched for gestational age, high-risk conditions, or obstetric management has yet been performed. Furthermore, it is unlikely that such a study will be initiated in the foreseeable future. Therefore, this section examines the limited available data concerning the roles of nonstress and contraction stress testing as primary indicators of fetal well-being.
Table 4 summarizes several studies in which the NST was used as a primary test and the CST was used as a backup or sequential test. The following conclusions can be drawn from these reports.
Table 4. NST as primary test and CST as backup test
Study (year) | No. Patients | CSTs Performed (%) | Sensitivity | Specificity | False-Positive Rate | False-Negative Rate |
Lee (1979) | 471 | 2.5 | 50 | 97 | 81 | 1 |
Keegan (1980) | 895 | 9 | 61 | 93 | 73 | 2 |
Phelan (1981) | 1452 | 16 | 45 | 82 | 89 | 3 |
Devoe (1985) | 281 | 5 | 50 | 100 | 7 | 6 |
The incidence of reactive tests associated with subsequently negative CSTs is nearly 100%. This implies that a reactive NST is an effective screening tool that can reduce the need for contraction stress testing, except in instances in which other FHR abnormalities, such as spontaneous decelerations or decreased long-term variability, require clarification.
The false negative rate of the NST is low and is not significantly different from that of the subsequent CST. This is presumptive support of the effectiveness of the reactive test alone in identifying uncompromised fetuses.
The false-positive rate of nonreactive tests is sufficiently high to support further testing with the CST.
Extending the length of the initial NST36, 37 for as long as 120 minutes may be useful in improving the classification of normal and abnormal fetuses. In one study,37 the incidence of pathologic CSTs following prolonged nonreactivity was 93%, implying that the absence of accelerations for 90 minutes or more in nonanomalous term fetuses is itself a strong sign of fetal compromise. Under these special circumstances, further testing with the CST may be unnecessary or ill advised if further fetal stress is to be avoided. Overall, the use of primary nonstress testing with selective CSTs has been shown to be an effective means of economizing testing efforts without reducing the diagnostic potential of FHR testing.
There have been few prospective comparative evaluations of both tests used as primary approaches in large patient populations. Of those reported, the collaborative study of Freeman and associates,102 involving 7448 patients, has suggested that both tests are effective in primary screening of high-risk patients but that antepartum death rates and perinatal morbidity (defined as IUGR, low Apgar score, and fetal distress), corrected for nonpreventable causes, were higher in the groups followed with NSTs. This study has been criticized for biases in patient selection (more patients were followed with CSTs, higher rates of low-birth-weight infants in the NST group), lack of standardization of test conditions or NST interpretation, and nonuniform follow-up of test results. More recently, Devoe and co-workers,122 reported a prospective collaborative study comparing the NST and nipple stimulation CST in 1270 patients. Two nearly identical study populations were matched for gestational age, incidence of high-risk indications, study conditions, NST interpretation, follow-up of abnormal tests, and end points for comparison. There were no significant differences in the perinatal mortality or morbidity rates, regardless of primary testing method. Although the sensitivity of the NST was somewhat higher than that of the nipple stimulation CST, it was also slightly more time-consuming to perform.
Because there are recognized diagnostic limitations in both primary approaches, the choice of testing method will ultimately be determined by the volume requirements of testing centers, the reliability of a given method in a specific population (often based on past performance), the presence or absence of contraindications to testing method, and the nature of the problem under consideration. At present, it remains likely that both tests will continue to be used as primary screening techniques in complicated pregnancy.
DIAGNOSTIC LIMITATIONS AND PITFALLS IN FHR TESTING
Reviews of the diagnostic performance of FHR testing have appeared in the recent literature, which suggest that, as screening instruments, both the NST and the CST have limitations.3, 4Table 5 and Table 6 summarize the sensitivities, specificities, and predictive values of selected major studies of both tests as related to perinatal mortality and morbidity.
Table 5. Diagnostic values (%) of NST and CST for perinatal morbidity
Study | No. Patients | Test | Sensitivity | Specificity | False-Positive | False-Negative |
Fox | 209 | CST | 22 | 90 | 40 | 10 |
Devoe | 297 | CST | 50 | 84 | 57 | 12 |
Keane | 566 | CST | 47 | 98 | 15 | 10 |
Krebs | 260 | CST | 55 | 99 | 14 | 7 |
Mendenhall | 367 | NST | 55 | 85 | 82 | 3 |
Devoe | 297 | NST | 52 | 82 | 78 | 5 |
Keane | 566 | NST | 53 | 88 | 54 | 10 |
Krebs | 253 | NST | 55 | 93 | 47 | 7 |
Weingold | 509 | NST | 38 | 90 | 89 | 2 |
Table 6. Diagnostic values (%) of NST and CST for perinatal mortality
Study | No. Patients | Test | Sensitivity | Specificity | False-Positive | False-Negative |
Devoe | 297 | CST | 33 | 85 | 98 | 1 |
Weingold | 381 | CST | 60 | 94 | 87 | 1 |
Keane | 566 | CST | 22 | 91 | 96 | 1 |
Freeman | 390 | CST | 43 | 85 | 85 | 4 |
Devoe | 297 | NST | 33 | 79 | 98 | 1 |
Evertson | 795 | NST | 67 | 63 | 97 | 1 |
Mendenhall | 367 | NST | 80 | 83 | 94 | 0 |
Keane | 566 | NST | 33 | 81 | 97 | 1 |
Both testing approaches are characterized by relatively high specificity (>90%) with wide ranges of sensitivity averaging 45–55%. False-negative rates fell below 10%, whereas false-positive rates exceeded 50%. The implications of such comparisons are that the NST and CST are significantly better at predicting the absence of fetal compromise than its presence, and abnormal NSTs or CSTs should be supported by other clinical data before obstetric intervention is undertaken. As indicated earlier, the addition of baseline reactivity assessment to the positive CST and extension of the length of observation for the nonreactive NST may improve this situation. Other possible remedies include the evaluation of long-term baseline variability,3 the addition of percentage acceleration time,123 and sequential comparison of tests in the same fetus.74
Reliability of a normal test
Reports of adverse perinatal outcomes following normal or reassuring FHR tests have appeared in the literature.34, 67, 75, 115, 116, 117, 118Table 7 is an aggregate summary of reports in which data are available to determine whether these outcomes could be considered preventable or nonpreventable through the standard testing approaches. Critical review of isolated cases of perinatal death suggest that the majority (approximately 60%) result from complications of preterm delivery, umbilical cord accidents, abruptio placentae, or congenital malformations and could be regarded as nonpreventable. Most of the remaining deaths occurred in pregnancies complicated by poorly controlled diabetes mellitus, postdatism, and IUGR. It is probable that further reduction in preventable deaths would require additional management measures, beyond FHR testing alone, such as improved medical care and closer fetal surveillance in selected pregnancies88 with the addition of ancillary diagnostic methods, such as biophysical profile testing.
Table 7. Cumulative reports of perinatal deaths following normal tests <7 days
Reported Mortality | Uncorrected Mortality Rate | Corrected* Mortality Rate |
Cumulative (NST): 80433 | 6.2/1000 | 2.5/1000 |
Cumulative (NST): 2154102 | 10.3/1000 | 4.2/1000 |
Cumulative (CST): 4626102 | 8.4/1000 | 3.5/1000 |
*Exclusions for congenital malformations, cord prolapse, sepsis, immaturity.
Reliability of an abnormal test
As implied earlier, a common shortcoming of both the NST and the CST is their relatively high false-positive rates. Given the limitations of arbitrary interpretative criteria, based on cutoff points or past clinical experience, such errors are inevitable. In a large longitudinal study of fetuses who exhibited a combination of nonreactivity, reduced variability, and spontaneous decelerations, Beischer and associates124 found that 93% of surviving infants available for follow-up had no major neurologic handicap. The consequences of misclassifying a normal infant may be serious (e.g., unwarranted preterm delivery, unindicated invasive procedures [amniocentesis] or cesarean deliveries, and excessive expenses and patient anxiety).
Pitfalls in the applications of the FHR tests may also reflect differences among observers, differing interpretative criteria, uncontrolled testing conditions, characteristics of the electronic monitoring equipment used, and inappropriate clinical responses to test outcomes. Studies of interobserver and intraobserver differences in the evaluation of FHR tests have isolated two key problems associated with their visual interpretations: inconsistency in evaluating pathologic tracings out of context,125 and decreased consistency among observers as the number of diagnostic categories is increased.126 Inadequate length of observation, failure to account for different standards related to gestational age, and nonstandardization of maternal status (e.g., drug administration, activity levels, and so on) may also confuse interpretation of subsequent FHR test patterns. Earlier-generation electronic monitors have been shown to produce greater signal loss during periods of fetal activity, spurious impressions of baseline variability, and less accurate baseline rates when compared with newer equipment.41, 127
Studies of effectiveness of NST as a primary test
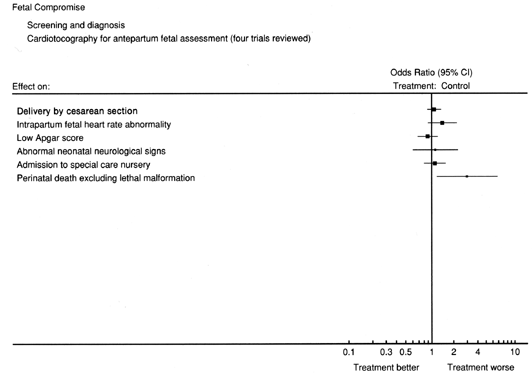
To date, there have been four randomized controlled clinical trials of NST as a primary screening method for high risk pregnancy.128, 129, 130, 131 While most current practices would perform testing more frequently than was the case in these trials (at least twice weekly as opposed to once weekly), it is interesting to note that NSTs were generally not the only well-being tools used. This raises unanswered questions regarding the impact of using NSTs on the outcomes measured. Figure 10 shows the measures studied and the odds-ratios of groups in which NST results were revealed to or concealed from clinicians.132 For only one outcome measure, corrected perinatal mortality, was there a significant difference between groups. While larger studies would certainly seem warranted, it is unlikely that any will be forthcoming.
|
More recently, our group compared the use of NST, amniotic fluid assessment, and umbilical artery Doppler velocimetry in 1000 high risk patients.133 This study demonstrated that initial pregnancy risk categories, that is, maternal hypertension and diabetes mellitus or fetal growth restriction and postdatism, were important modifiers of test reliability. The value of individual tests in assessing risk of fetal compromise varied not only according to risk category but also according to test selected. No single testing method, including the NST, appeared to be sufficiently sensitive to fetal compromise for most risk categories. Best screening performances (sensitivities >70%) were obtained for the NST only with the categories of fetal growth or maternal hypertension.
It is important to maintain proper perspective on the place of FHR testing in overall clinical management. Such approaches were intended to assist high risk care but not to substitute for contextual evaluation using all available data. Retrospective studies of the NST by Platt and associates134 and of the CST by Garite and colleagues135 indicate that the use of FHR testing has been linked to a significant reduction in antepartum fetal deaths. FHR testing, especially the NST, remains an important component of antepartum care, although current information would suggest that it should not be considered as a “stand-alone” test. Developments in the areas of automated analysis, actocardiography, and objective clinical archives should lead to improved and more appropriate use of FHR testing.
CONCLUDING REMARKS
There has been little substantive research and development in the past on antepartum fetal heart rate testing. Partly, this can be attributed to the increasing use of real-time ultrasonography in augmenting fetal assessment, as in the biophysical profile or BPP. The NST has been included as one of five components in such testing schemes and has become less of a 'stand alone' assessment method. While the CST remains a very robust test of placental well-being, its logistic constraints and time requirements have led to its gradual replacement by either complete BPP testing (all five components) or modified BPP testing (NST and amniotic fluid volume assessment). The lack of more recent prospective studies of either fetal heart rate testing is unfortunate since a number of questions remain incompletely answered. These include the optimal timing of test initiation, post-test risk adjustment for specific high risk conditions, and testing of fetuses between 24 and 32 weeks' gestation. This latter issue has become increasingly important as the threshold of fetal viability has been extended downward since the introduction of fetal heart rate testing in the 1970s. Finally, although evidence-based medicine has been applied extensively to many screening and diagnostic modalities, high quality randomized controlled trials for fetal heart testing have been conspicuously lacking. Hopefully, some of these important concerns will be revisited by future investigators.
REFERENCES
Pose SV, Castillo JB, Nora-Rojas EO et al: Test of fetal tolerance to induce uterine contractions for the diagnosis of chronic distress. In: Perinatal Factors Affecting Human Development, p 96. Washington, DC, Pan American Health Organization, 1969 |
|
Kubli FW, Kaeser O, Hinselmann M: Diagnostic management of chronic placental insufficiency. In Pecile A, Finzi C (eds): The Foeto-Placental Unit, p 323. Amsterdam, Excerpta Medica Foundation, 1969 |
|
Devoe LD, Castillo RA, Sherline DM: The nonstress test as a diagnostic test: A critical reappraisal. Am J Obstet Gynecol 152: 1047, 1985 |
|
Thacker SB, Berkelman RL: Assessing the diagnostic accuracy and efficacy of selected antepartum fetal surveillance techniques. Obstet Gynecol Surv 41: 121, 1986 |
|
Dalton KJ, Dawes GS, Patrick JE: The autonomic nervous system and fetal heart rate variability. Am J Obstet Gynecol 146: 456, 1983 |
|
Martin CB Jr: Regulation of the fetal heart rate and genesis of FHR patterns. Semin Perinatol 2: 131, 1978 |
|
Murata Y, Martin CB, Ikenoue T et al: Fetal heart rate accelerations and late decelerations during the course of intrauterine death in chronically catheterized rhesus monkeys. Am J Obstet Gynecol 144: 218, 1982 |
|
Bocking AD, Harding R, Wickham PJD: Effects of reduced uterine blood flow on accelerations and decelerations in heart rate of fetal sheep. Am J Obstet Gynecol 154: 329, 1986 |
|
Itskovitz J, LaGamma EF, Rudolph AM: Heart rate and blood pressure responses to umbilical cord compression in fetal lambs with special reference to mechanism of variable decelerations. Am J Obstet Gynecol 147: 451, 1983 |
|
Natale R, Nasello-Patterson C, Turok R: Longitudinal measurements of fetal breathing, body movements, heart rate, and heart rate accelerations and decelerations at 24 to 32 weeks of gestation. Am J Obstet Gynecol 151: 256, 1985 |
|
Sorokin Y, Pillay SK, Dierker LJ et al: The association between fetal heart rate patterns and fetal movements in pregnancies between 20 and 30 weeks' gestation. Am J Obstet Gynecol 143: 243, 1982 |
|
Aladjem S, Vuolo K, Pazos R et al: Antepartum fetal testing: Evaluation and redefinition of criteria for clinical interpretation. Semin Perinatol 5: 145, 1981 |
|
Patrick J, Carmichael L, Chen L et al: Accelerations of the human fetal heart rate at 38 to 40 weeks' gestational age. Am J Obstet Gynecol 148: 35, 1984 |
|
Martin CB: Behavioral states in the human fetus. J Reprod Med 26: 425, 1981 |
|
Assali NS, Brinkman CR, Woods JR et al: Development of neurohumoral control of fetal, neonatal and adult cardiovascular function. Am J Obstet Gynecol 129: 748, 1977 |
|
Campbell K: Ultradian rhythms in the fetus during the last 10 weeks gestation: A review. Semin Perinatol 4: 301, 1980 |
|
Visser GHA, Goodman JDS, Levine DH et al: Diurnal and other cyclic variations in human fetal heart rate near term. Am J Obstet Gynecol 142: 535, 1982 |
|
Dalton KJ, Dawes GS, Patrick JE: Diurnal, respiratory and other rhythms of fetal heart rate in lambs. Am J Obstet Gynecol 127: 414, 1977 |
|
Patrick J, Campbell K, Carmichael L et al: Patterns of fetal gross body movements over 24-hour observation intervals during the last 10 weeks of pregnancy. Am J Obstet Gynecol 142: 363, 1982 |
|
Gelman SR, Spellacy WN, Wood S et al: Fetal movements and ultrasound: Effect of maternal intravenous glucose administration. Am J Obstet Gynecol 137: 459, 1980 |
|
Richardson B, Briggs ML, Toomey C et al: The effect of maternal glucose administration on the specificity of the nonstress test. Am J Obstet Gynecol 137: 459, 1980 |
|
Artal R, Rutherford S, Romen Y et al: Fetal heart rate responses to maternal exercise. Am J Obstet Gynecol 155: 729, 1986 |
|
Devoe LD, Arthur M, Searle N: The effects of maternal ambulation on the nonstress test. Am J Obstet Gynecol 157: 240, 1987 |
|
Rayburn WF, Motley ME, Zuspan FP: Conditions affecting nonstress test results. Obstet Gynecol 59: 490, 1982 |
|
Devoe LD, O'Dell BE, Castillo RA et al: Metastic pheochromocytoma in pregnancy: The fetal biophysical effects following maternal administration of alpha-adrenergic, beta-adrenergic, and dopamine antagonists. Obstet Gynecol 68 (3 Suppl): 15S, 1986 |
|
Margulis E, Binder D, Cohen DW: The effect of propranolol on the nonstress test. Am J Obstet Gynecol 133: 579, 1979 |
|
Keegan KA, Paul RH, Broussard PM et al: Antepartum fetal heart rate testing: III. The effect of phenobarbital on the nonstress test. Am J Obstet Gynecol 133: 579, 1979 |
|
Quigley ME, Sheehan KL, Wilkes MM et al: Effects of maternal smoking on circulating catecholamine levels and fetal heart rates. Am J Obstet Gynecol 133: 685, 1979 |
|
Peeters LL, Shelton RE, Jones MD et al: Blood flow to fetal organs as a function of arterial oxygen content. Am J Obstet Gynecol 135: 637, 1979 |
|
Sheldon RE, Peeters LLH, Jones MD et al: Redistribution of cardiac output and oxygen delivery in the hypoxemic fetal lamb. Am J Obstet Gynecol 135: 1071, 1979 |
|
Nathanielsz PW, Bailey A, Poore ER et al: The relationship between myometrial activity and sleep state and breathing in fetal sheep throughout the last third of gestation. Am J Obstet Gynecol 138: 653, 1980 |
|
Powell OH, Melville A, MacKenna JL: Fetal heart rate acceleration in labor: Excellent prognostic indicator. Am J Obstet Gynecol 134: 36, 1979 |
|
Hammacher K: The clinical significance of cardiotocography. In Huntingford P, Hunter M, Saling E (eds): Perinatal Medicine, pp 80–93. New York, Academic Press, 1970 |
|
Lee CY, Diloreto PC, O'Lane JM: A study of fetal heart rate acceleration patterns. Obstet Gynecol 45: 142, 1975 |
|
Rochard F, Schifrin BS, Goupil F et al: Nonstressed fetal heart rate monitoring in the antepartum period. Am J Obstet Gynecol 126: 699, 1976 |
|
Brown R, Patrick J: The nonstress test: How long is enough? Am J Obstet Gynecol 141: 646, 1981 |
|
Devoe LD, McKenzie J, Searle NS et al: Clinical sequelae of the extended nonstress test. Am J Obstet Gynecol 151: 1074, 1985 |
|
Devoe LD: Antepartum fetal heart rate testing in pre-term pregnancy. Obstet Gynecol 60: 431, 1982 |
|
Druzin ML, Fox A, Kofut E et al: The relationship of the nonstress test to gestational age. Am J Obstet Gynecol 153: 386, 1985 |
|
Smith CV, Phelan JP, Paul RH: A prospective analysis of the influence of gestational age on the baseline fetal heart rate and reactivity in a low risk population. Am J Obstet Gynecol 153: 780, 1985 |
|
Lawson GW, Belcher R, Dawes GS et al: A comparison of ultrasound (with autocorrelation) and direct electrocardiogram fetal heart rate detector systems. Am J Obstet Gynecol 147: 721, 1983 |
|
Divon MY, Torres FP, Yeh SY et al: Autocorrelation techniques in fetal monitoring. Am J Obstet Gynecol 151: 2, 1985 |
|
Klapholz H: Techniques of fetal heart rate monitoring. Semin Perinatol 2: 119, 1978 |
|
Neldam S, Jessen P: Fetal movements registered by the pregnant women correlated to retrospective estimations of fetal movements from cardiotocographic tracings. Am J Obstet Gynecol 136: 1051, 1980 |
|
Devoe LD, McKenzie J, Searle N et al: Nonstress test: Dimensions of normal reactivity. Obstet Gynecol 66: 617, 1985 |
|
Visser GHA, Zeelenberg HJ, DeVries JIP et al: External physical stimulation of the human fetus during episodes of low heart rate variation. Am J Obstet Gynecol 145: 579, 1983 |
|
Read JA, Miller FC: Fetal heart rate acceleration in response to acoustic stimulation as a measure of fetal well-being. Am J Obstet Gynecol 129: 512, 1977 |
|
Smith CV, Phelan JP, Broussard PM, Paul RH: Fetal acoustic stimulation testing. A retrospective analysis of the fetal acoustic stimulation test. Am J Obstet Gynecol 153: 567, 1985 |
|
Smith CV, Phelan JP, Platt LD et al: Fetal acoustic stimulation testing. II. A randomized comparison with the nonstress test. Am J Obstet Gynecol 155: 131, 1986 |
|
Gagnon R, Hunse C, Patrick J: Fetal responses to vibratory acoustic stimulation: Inflection of basal heart rate. Am J Obstet Gynecol 159: 835, 1988 |
|
Devoe LD, Searle NA, Ruedrich DA et al: The effects of vibroacoustic stimulation on baseline heart rate, breathing activity, and body movements of normal term fetuses. Am J Obstet Gynecol 161: 524, 1989 |
|
Devoe LD, Gardner P, Arnold P, Searle BA: The effects of vibratory acoustic stimulation on baseline fetal heart rate in term pregnancy. Am J Obstet Gynecol 160: 1086, 1989 |
|
Smith CV, Satt R, Phelan JP, Paul RH: Intrauterine sound levels: Intrapartum assessment within intrauterine environment. Am J Perinatol 7: 312, 1990 |
|
Nyman M, Barr M, Westgren M: A four-year followup of hearing and development in children exposed in utero to vibroacoustic stimulation. Br J Obstet Gynecol 99: 685, 1992 |
|
Maeda K: Studies on new ultrasonic doppler fetal actograph and continuous recordings of fetal movement. Acta Obstet Gynaecol Jpn 36: 280, 1984 |
|
Schmidt W, Gnirs J: Das KCTG-erste klinische Erfahrungen beim, Einsatz des Kinetocardiotokogramms. Geburtshilfe Frauenheilkd 51: 437, 1991 |
|
Besinger RE, Johnson TRB: Doppler recordings of fetal movement: Clinical correlation with realtime ultrasound. Obstet Gynecol 74: 277, 1989 |
|
Melendez TD, Rayburn WF, Smith CV: Characterization of fetal body movement recorded by Hewlett Packard M1350A fetal monitor. Am J Obstet Gynecol 167: 700, 1992 |
|
Stanco LM, Rabello Y, Medearis AL et al: Does doppler-detected fetal movements decrease the incidence of nonstress tests? Obstet Gynecol 82: 999, 1993 |
|
Devoe L, Boehm F, Paul R et al: Clinical experience with the Hewlett Packard M-1350 fetal monitor: Correlation of doppler-detected fetal body movements with fetal heart rate parameters and perinatal outcome. Am J Obstet Gynecol 170: 650, 1994 |
|
Searle JR, Devoe LD, Phillips MC, Searle NS: Computerized analysis of resting fetal heart rate tracings. Obstet Gynecol 71: 407, 1988 |
|
Dawes GS, Moulden M, Redman CWG: System 8000: Computerized antenatal FHR analysis. J Perinat Med 19: 47, 1991 |
|
Schneider E, Schulman H, Farmakieds G, Paksima S: Comparison of the interpretation of antepartum fetal heart rate tracings between a computer program and experts. J Mat Fet Invest 1: 205, 1991 |
|
Hiett AK, Devoe LD, Youssef A et al: A comparison of visual and automated methods of analyzing fetal heart rate tests. Am J Obstet Gynecol 168: 1517, 1993 |
|
Blumkofe KA, Broussard PM, Walla CA, Platt LD: Computerized versus visual analysis of fetal heart rate: A reduction in testing time. Am J Obstet Gynecol 166: 415, 1992 |
|
Evertson LR, Gautier RJ, Schifrin BS et al: Antepartum fetal heart rate testing: I. Evolution of the nonstress test. Am J Obstet Gynecol 133: 29, 1979 |
|
Keegan KA, Paul RH: Antepartum fetal heart rate testing: IV. The nonstress test as a primary approach. Am J Obstet Gynecol 136: 75, 1980 |
|
Mendenhall HW, O'Leary JA, Phillips KO: The nonstress test: The value of a single acceleration in evaluating the fetus at risk. Am J Obstet Gynecol 136: 87, 1980 |
|
Visser GHA, Huisjes HS: Diagnostic value of the unstressed antepartum cardiotocogram. Br J Obstet Gynaecol 84: 321, 1977 |
|
Krebs HB, Petres RE: Clinical application of a scoring system for evaluation of antepartum fetal heart rate monitoring. Am J Obstet Gynecol 130: 765, 1978 |
|
Lyons ER, Bylsma-Howell M, Siamsi S et al: A scoring system for nonstressed antepartum fetal heart rate monitoring. Am J Obstet Gynecol 133: 242, 1979 |
|
Devoe LD, Yanowitch G, Azor H: The application of multiple-parameter scoring to antepartum fetal heart rate testing. J Reprod Med 26: 250, 1981 |
|
Pearson JF, Weayer JB: A six-point scoring system for antenatal cardiotocographs. Br J Obstet Gynaecol 85: 321, 1978 |
|
Devoe LD, Castillo R, McKenzie J et al: Sequential non-stress testing using each fetus as its own control. Am J Obstet Gynecol 154: 931, 1986 |
|
Schifrin BS, Foye G, Amato J et al: Routine fetal heart rate monitoring in the antepartum period. Obstet Gynecol 54: 21, 1979 |
|
Elynn AM, Kelly J, O'Connor M: Unstressed antepartum cardiotocography in the management of the fetus suspected of growth retardation. Br J Obstet Gynaecol 86: 106, 1979 |
|
Lin CC, Devoe LD, River P et al: Oxytocin challenge test and intrauterine growth retardation. Am J Obstet Gynecol 140: 282, 1981 |
|
Devoe LD, Sholl JS: Postdates pregnancy: Assessment of fetal risk and obstetric management. J Reprod Med 28: 576, 1983 |
|
Yeh SY, Read JA: Management of post-term pregnancy in a large obstetric population. Obstet Gynecol 60: 282, 1982 |
|
Lavin JP, Miodovnik M, Barden TP: Relationship of nonstress test reactivity and gestational age. Obstet Gynecol 63: 338, 1984 |
|
Bailey D, Flynn AM, Kelly J et al: Antepartum fetal heart rate monitoring in multiple pregnancy. Br J Obstet Gynaecol 87: 561, 1980 |
|
Devoe LD, Azor H: Simultaneous nonstress fetal heart rate testing in twin pregnancy. Obstet Gynecol 58: 450, 1981 |
|
Blake GD, Knuppel RA, Ingardia CJ et al: Evaluation of nonstress testing in multiple gestations. Obstet Gynecol 63: 528, 1984 |
|
Nayot D, Mor-YoSef S, Granat M et al: Antepartum fetal heart rate pattern associated with major congenital malformations. Obstet Gynecol 63: 414, 1984 |
|
Powell-Phillips WD, Towell ME: Abnormal fetal heart rate associated with congenital abnormalities. Br J Obstet Gynaecol 87: 270, 1980 |
|
Vorher H: Placental insufficiency in relation to post-term pregnancy and fetal postmaturity. Am J Obstet Gynecol 123: 67, 1975 |
|
Young BK, Katz M, Wilson SJ: Sinusoidal fetal heart rate: I. Clinical significance. Am J Obstet Gynecol 136: 587, 1980 |
|
Boehm FH, Salyer S, Shah DM et al: Improved outcome of twice weekly nonstress testing. Obstet Gynecol 67: 566, 1986 |
|
Ray M, Freeman RK, Pine S et al: Clinical experience with the oxytocin challenge test. Am J Obstet Gynecol 114: 1, 1972 |
|
Freeman RK: The use of the oxytocin challenge test for antepartum clinical evaluation of uteroplacental respiratory function. Am J Obstet Gynecol 121: 481, 1975 |
|
Trierweiler MW, Freeman RK, James J: Baseline fetal heart rate characteristics as an indicator of fetal status during the antepartum period. Am J Obstet Gynecol 125: 618, 1976 |
|
Fox HE, Steinbrecher M, Ripton B: Antepartum fetal heart and uterine activity studies: I. Preliminary report of accelerations and the oxytocin challenge test. Am J Obstet Gynecol 126: 61, 1976 |
|
Farahani G, Fenton AN: Fetal heart rate acceleration in relation to the oxytocin challenge test. Obstet Gynecol 49: 163, 1977 |
|
Braly P, Freeman RK: The significance of fetal heart rate reactivity with a positive oxytocin challenge test. Obstet Gynecol 50: 689, 1977 |
|
Huddleston JF, Sutliff G, Robinson D: Contraction stress test by intermittent nipple stimulation. Obstet Gynecol 63: 699, 1984 |
|
Devoe LD: Clinical implications of prospective antepartum fetal heart rate testing. Am J Obstet Gynecol 137: 983, 1980 |
|
Keane MW, Horger ED, Vice L: Comparative study of stressed and nonstressed antepartum fetal heart rate testing. Obstet Gynecol 57: 320, 1981 |
|
Lee CY, Drukker B: The nonstress test for the antepartum assessment of fetal reserve. Am J Obstet Gynecol 134: 460, 1979 |
|
Leveno KJ, Williams ML, DePalma RT et al: Perinatal outcome in the absence of antepartum fetal heart rate acceleration. Obstet Gynecol 61: 347, 1983 |
|
Phelan JP: The nonstress test: A review of 3000 tests. Am J Obstet Gynecol 139: 7, 1981 |
|
Mashini IS, Devoe LD, McKenzie J et al: Comparison of uterine activity induced by nipple stimulation and oxytocin. Obstet Gynecol 69: 74, 1987 |
|
Freeman RK, Anderson G, Dorchester W: A prospective multi-institutional study of antepartum fetal heart rate monitoring: I. Risk of perinatal mortality and morbidity according to antepartum fetal heart rate test results. Am J Obstet Gynecol 143: 771, 1982 |
|
Devoe LD: Clinical features of the reactive positive contraction stress test. Obstet Gynecol 63: 523, 1984 |
|
Staisch KJ, Westlake JR, Bashore RA: Blind oxytocin challenge test and perinatal outcome. Am J Obstet Gynecol 138: 399, 1980 |
|
Baskett TF, Sandy EA: Oxytocin challenge test and antepartum fetal assessment. Br J Obstet Gynaecol 84: 39, 1977 |
|
Schifrin BS, Lapidus M, Doctor GS et al: Contraction stress for antepartum fetal evaluation. Obstet Gynecol 45: 433, 1975 |
|
Trimbos JB, Keirse MJNC: Significance of antepartum cardiotocography in normal pregnancy. Br J Obstet Gynaecol 85: 907, 1978 |
|
Phelan JP, Lewis PE: Fetal heart rate decelerations during a nonstress test. Am J Obstet Gynecol 57: 228, 1981 |
|
O'Leary JA, Adrinopoulous GC, Gioroano PC: Variable decelerations and the nonstress test: An indication of cord compromise. Am J Obstet Gynecol 137: 704, 1980 |
|
Pazos R, Vuolo K, Aladjem S et al: Association of spontaneous fetal heart rate decelerations during antepartum nonstress testing and intrauterine growth retardation. Am J Obstet Gynecol 144: 574, 1982 |
|
Bourgeois FJ, Thiagarajah S, Harbert GM: The significance of fetal heart rate decelerations during nonstress testing. Am J Obstet Gynecol 150: 213, 1984 |
|
Druzin ML, Gratacos J, Keegan K, Paul RH: Antepartum fetal heart rate testing: VII. The significance of fetal bradycardia. The significance of fetal bradycardia. Am J Obstet Gynecol 139: 194, 1981 |
|
Odendaal HJ: Hyperstimulation of the uterus during the oxytocin stress test. Obstet Gynecol 51: 380, 1978 |
|
Gabbe SG, Freeman RK, Goebelsmann U: Evaluation of the contraction stress test before 33 weeks' gestation. Obstet Gynecol 52: 649, 1978 |
|
Klapholz H, Burke L: Intrauterine fetal demise with a negative oxytocin challenge test. J Reprod Med 15: 169, 1975 |
|
Lorenz RP, Pagano JS: A case of fetal death after a negative oxytocin challenge test. Am J Obstet Gynecol 130: 232, 1978 |
|
Salerno NJ, Thomas RK: A further challenge to the validity of the weekly interval between oxytocin challenge tests. Am J Obstet Gynecol 130: 232, 1978 |
|
Evertson LR, Gauthier RJ, Collea JV: Fetal demise following negative contraction stress tests. Obstet Gynecol 51: 671, 1978 |
|
Liggins GC, Howie RN: A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics 50: 515, 1972 |
|
Bissonnette JM, Johnson K, Toomey C: The role of a trial of labor with a positive contraction stress test. Am J Obstet Gynecol 135: 292, 1979 |
|
Odendaal NJ: The fetal and labor outcome of 102 positive contraction stress tests. Obstet Gynecol 54: 591, 1979 |
|
Devoe KD, Morrison J, Martin J et al: A prospective comparative study of the extended nonstress test and nipple stimulation contraction stress test. Am J Obstet Gynecol 157: 531, 1987 |
|
Devoe LD, Castillo R, Saad S et al: Percent acceleration time: A new method of fetal assessment. Obstet Gynecol 67: 191, 1986 |
|
Beischer BA, Drew JH, Ashton PW et al: Quality of survival of infants with critical fetal reserve detected by antenatal cardiotocography. Am J Obstet Gynecol 146: 662, 1983 |
|
Trimbos JB, Keirse MJ: Observer variability in assessment of antepartum cardiotocograms. Br J Obstet Gynaecol 85: 900, 1978 |
|
Hage ML: Interpretation of nonstress tests. Am J Obstet Gynecol 153: 490, 1985 |
|
Boehm FH, Fields LM, Hutchinson JM et al: The indirectly obtained fetal heart rate: Comparison of first and second generation electronic fetal monitors. Am J Obstet Gynecol 155: 10, 1986 |
|
Brown V, Sawers RS, Parsons RJ et al: The value of antenatal cardiotocography in the management of high risk pregnancy: A randomized controlled trial. Br J Obstet Gynaecol 89: 716, 1982 |
|
Flynn AM, Kelly J, Mansfield H et al: A randomized controlled trial of nonstress antepartum cardiotocography. Br J Obstet Gynaecol 89: 427, 1982 |
|
Kidd LC, Patel NB, Smith R: Nonstress antenatal cardiotocography—a prospective randomized clinical trial. Br J Obstet Gynaecol 92: 1156, 1985 |
|
Lumley J, Lester A, Anderson I: A randomized trial of weekly cardiotocography in high risk obstetric patients. Br J Obstet Gynaecol 90: 1018, 1983 |
|
Neilson JP: Cardiotocography for antepartum fetal assessment in pregnancy and childbirth module. In Enkin MW, Keirse MJNC, Renfrew MJ, Neilson JP (eds): Cochrane Database of Systematic Reviews. Review no. 03881, March 24, 1993. Disk Issue 1. Oxford, Update Software, 1994 |
|
Devoe LD, Gardner P, Dear C, Castillo RA: The diagnostic values of concurrent nonstress testing, amniotic fluid measurement, and doppler velocimetry in screening a general high risk population. Am J Obstet Gynecol 163: 1040, 1990 |
|
Platt LD, Paul RH, Phelan J et al: Fifteen years of experience with antepartum fetal testing. Am J Obstet Gynecol 156: 1509, 1987 |
|
Garite TJ, Freeman RK, Hochleutner I et al: Oxytocin challenge test: Achieving the desired goals. Obstet Gynecol 51: 614, 1978 |